Recent learnings in treating MASH

Transcript
WOMAN: In March of 2024, resmetirom became the first therapy approved by the FDA
to treat adult patients with MASH with moderate to severe fibrosis. [MUSIC PLAYING]
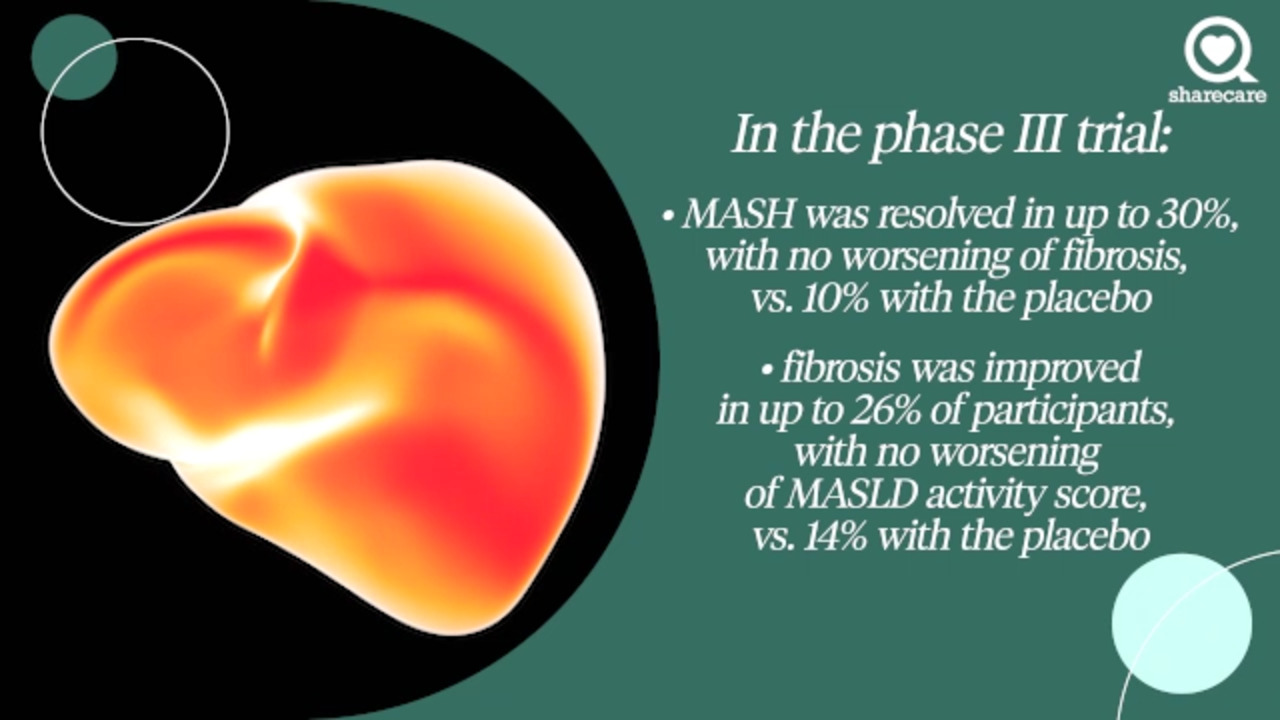
In a phase III trial with approximately 1,000 participants, 80- and 100-milligram doses of resmetirom were tested against placebo.
MASH was resolved in up to 30% without worsening fibrosis, versus 10% in the placebo group.
Fibrosis was improved in up to 26% of participants with no worsening of MASLD activity
versus 14% with the placebo. At both doses, cholesterol levels also declined.
Adverse events in the trial were reported at similar rates across all three groups, ranging from 10.9% to 12.7%.
liver health
Browse videos by topic categories
A
B
C
D
E
F
G
H
I
J
K
L
M
N
O
P
Q
R
S
T
U
V
W
X
Y
Z
ALL