Updated on December 16, 2024.
When COVID began spreading around the world in early 2020, there were no effective treatments. However, the world’s leading scientists and health agencies worked together in an extraordinary collaboration during the first few years of the pandemic to develop not just life-saving vaccines but also medications and treatments that could help prevent and ease severe symptoms.
And even now, as the virus that causes COVID, known as SARS-CoV-2, evolves and mutates, updated vaccines and newer treatments may continue to be developed over time.
Frontline prevention: vaccines
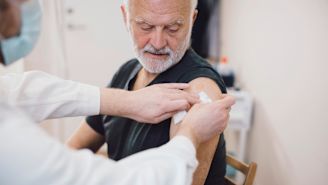
COVID vaccines are safe, effective, and vital for preventing severe illness, hospitalization, or death from the disease. Everyone ages 6 months and older should receive the latest updated COVID-19 vaccine (produced by Pfizer-BioNTech, Moderna, or Novavax, as these are re-issued to protect against new variants of the virus), according to the Centers for Disease Control and Prevention (CDC).
Children ages 6 months to 5 years old, people age 65 and older, and those who are immunocompromised may need additional doses of the latest vaccines.
Other preventive medications
For some people who are at risk of getting a severe case of COVID, in March 2024 the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for a medication called pemivibart (Pemgarda). This is a preventive monoclonal antibody, which means it contains molecules created by scientists in the lab that imitate the immune system’s natural antibodies.
Pemivibart is available for anyone who is at least 12 years old, weighs at least 88 pounds, and is moderately or severely immunocompromised because of immunosuppressive medication or a medical condition.
Pemivibart is not a replacement for vaccination. Instead, it’s an added layer of protection that can be given two weeks after having received a COVID vaccine. It comes in an intravenous infusion that takes 60 minutes to be administered by a healthcare provider (HCP). It can be given once every three months, as needed.
If you’re privately insured or have Medicare Part B, you may be able to receive pemivibart at no cost.
Managing a COVID infection
Be sure to stay at home if you have COVID to avoid spreading it to others. If you’re infected, you also have many options for treatment, depending on your risk level. People at high risk of severe infections include unvaccinated people, adults age 65 and older, people who are not up to date on vaccinations, and people with medical conditions that affect their lungs, heart, or immune system.
If you have mild-to-moderate symptoms and are at low risk: The same treatments that works for colds and flu can help bring you relief, including over-the-counter (OTC) medicines like ibuprofen (Advil, Motrin, etc), acetaminophen (Tylenol, Aleve, etc), and cough syrup.
If you have mild-to-moderate symptoms and are at high risk: You may also be eligible for antivirals. These treatments, which can be taken by mouth or intravenously, work by stopping the virus from making copies of itself.
Nirmatrelvir with ritonavir (Paxlovid) and molnupiravir (Lagevrio) are pills that can be taken up to five days after symptoms first show up, though they should ideally be taken as soon symptoms develop. While nirmatrelvir with ritonavir can be taken by those who are 12 years and older and weigh at least 88 pounds, molnupiravir can only be taken by those older than 18 who have no other treatment options.
Remdesivir (Veklury) is an infusion antiviral that can be taken up to seven days after the first symptoms appear, though again, ideally it should be taken as soon as there are signs of COVID. Remdesivir can be taken by people of almost any age, except newborns. A person must be at least 28 days old and weigh at least seven pounds and be at high risk of getting very ill from COVID. They can be either an outpatient or an inpatient at the hospital.
Most antivirals require a prescription from an HCP, but nirmatrelvir with ritonavir can also be prescribed by a pharmacist licensed by the state.
Recovering from COVID
If you’ve received treatment (either prescription or OTC), your symptoms have improved, and you’ve gone 24 hours without a fever (and aren’t taking any fever-reducing medicines), then you are likely safe to re-enter everyday life and be around others.
However, to help prevent the spread of the virus, you should wear a mask for the next five days along with continuing to be mindful about hygiene, including frequent handwashing and covering your sneezes and coughs.
How to protect your health
It’s important not to take any prescription or alternative medications to treat COVID that haven’t been prescribed to you specifically for that purpose. If you’re thinking of taking a medication recommended by a friend or by the author of an online article, put your health and safety first and consult your HCP.